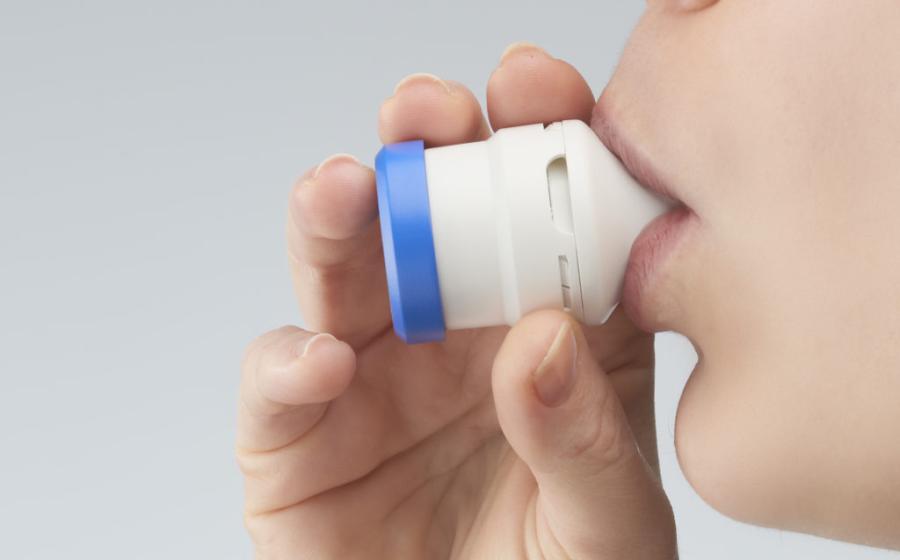
Aptar Pharma, a global leader in drug delivery and active material science solutions and services, today announced that it has acquired the worldwide rights to clinical stage drug developer Pharmaxis’ proprietary Orbital™ inhaler, a unique device designed to deliver high payload dry powder to the lungs. The Orbital technology helps meet an increasing global need to deliver high doses of drugs, such as antibiotics, to the lungs and allows powder payloads of up to 400mg to be inhaled by patients in divided doses without the need to reload. This unique platform was originally developed as a life cycle extending product for the Pharmaxis cystic fibrosis drug Bronchitol® (mannitol).
This acquisition strengthens Aptar Pharma’s leadership in respiratory by offering a broader range of devices and services, from formulation to patient. Coupled with their formulation expertise at Nanopharm, Human Factors and patient onboarding at Noble, analytical services at Next Breath – all Aptar Pharma companies - as well as their Digital Health offering, Aptar Pharma is able to optimize device design, customize drug development and differentiate with improved compliance to provide their customers with a value-added finished product.