More than 50,000 containers of a widely sold pain-relief ointment have been recalled after federal officials warned the packaging could put young children at risk of serious injury or death if accidentally swallowed.
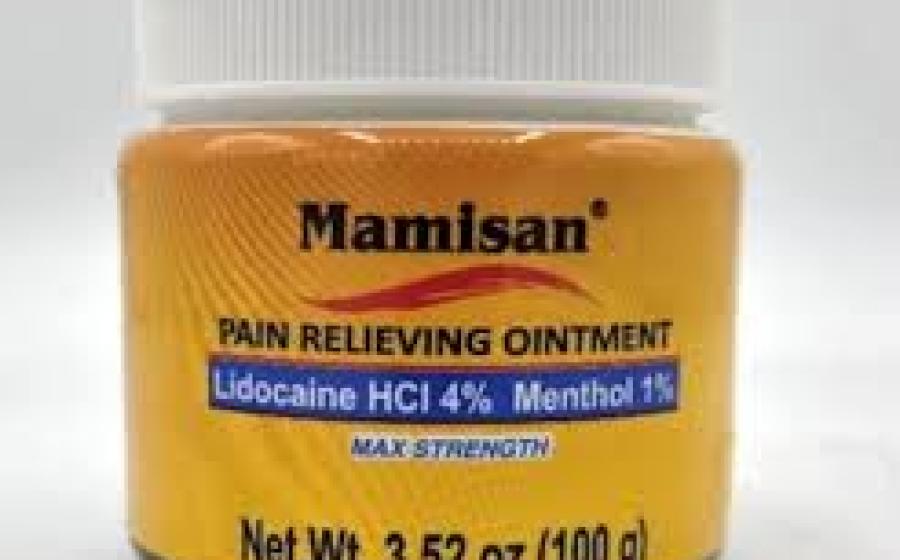
Plantimex Distributors announced the recall of its Mamisan Pain Relieving Topical Ointment after determining the product’s lid failed to meet child-resistant requirements under the Poison Prevention Packaging Act, according to a notice published by the U.S. Consumer Product Safety Commission.
The ointment contains lidocaine, a numbing agent that must be sold in child-resistant packaging. Regulators said the jars, sold in bright orange containers with a white twist-off lid, do not provide that protection and pose a poisoning hazard if accessed by children.
About 50,330 units are affected by the recall. The product was sold at Walmart and Target stores nationwide, as well as online at Target.com, from April 2024 through October of this year for about $10. Only jars labeled with UPC code 860006498115 are included.
No injuries have been reported, officials said, but consumers are urged to immediately place the ointment out of children’s reach and contact Plantimex for a free replacement lid designed to meet federal safety standards. Once the new lid is installed, the product can be used safely as directed, the company said.
The ointment was manufactured by MiramarLab of Doral, Florida, and distributed by Plantimex Distributors of San Diego.