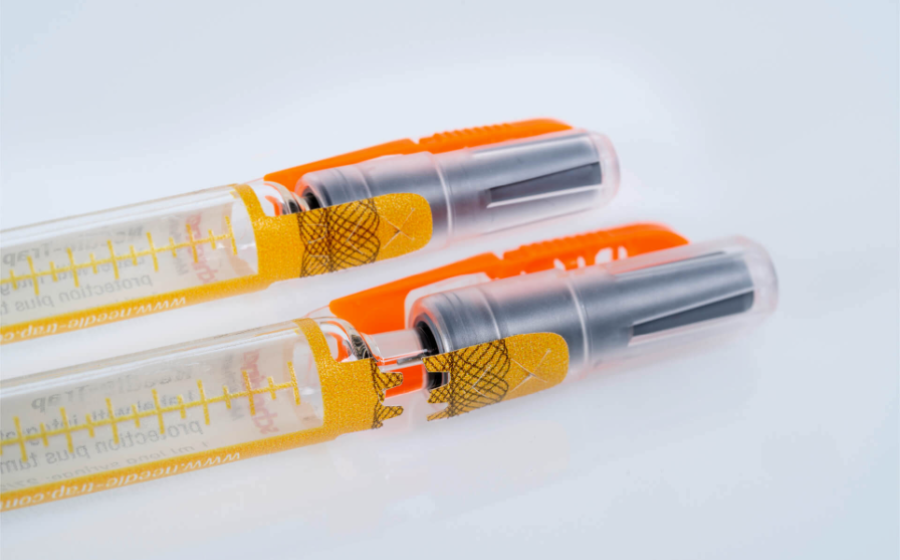
A secure supply chain and the integrity of medicine packaging are essential for pharmaceutical manufacturers. Regulations such as the EU Falsified Medicines Directive support that need but only address secondary packaging. Primary containers, however, require more specialized solutions for tamper evidence and first-opening indication. Due to its integrated plastic trap, Needle-Trap poses special challenges concerning first-opening indication. Smooth processing of the label on dispensing systems in pharmaceutical manufacturing environments and ease of use in healthcare settings must be considered.
Easy Process Integration–Safe Use
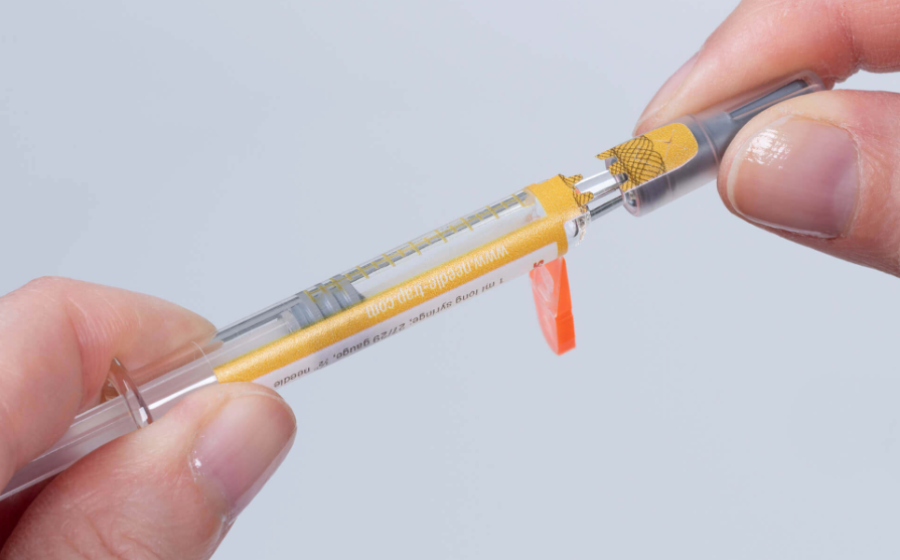
The development features a closure seal integrated into the syringe protection label with a tab ending on the syringe cap. Prior to the injection, the needle trap is folded sideways as usual. While the cap is being pulled off a perforation triggers the activation of the first-opening seal. Security cuts additionally prevent undetected removal of the seal. The closure seal can also be complemented by overt authentication features, such as a guilloche, or covert security features for authenticity verification. Pharmaceutical manufacturers are provided with a multifunctional and cost-efficient solution that can be integrated easily into existing production processes and enhances the product and patient safety. The integrated seal can even replace blister packs for integrity protection on the unit level. Healthcare staff benefits from efficient and reliable needle protection as well as from convenient first-opening indication that is irreversible and detectable at first glance.




