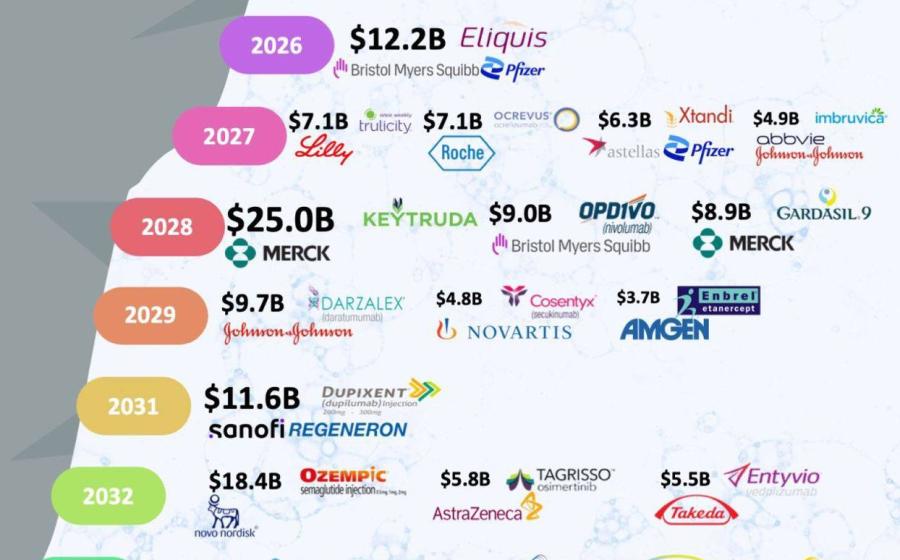
As the pharmaceutical industry braces for a new wave of blockbuster drugs going off-patent—a phenomenon known as the "patent cliff"—the immediate conversation usually revolves around declining revenues for originators and opportunities for generic manufacturers. But beyond drug formulation and distribution, there lies a strategic, often overlooked opportunity: packaging, labeling, and artwork management.
What is the Patent Cliff?
The patent cliff refers to the expiration of patents on high-revenue pharmaceutical products, opening the door for generic competition. Between 2024–2030, several billion-dollar drugs—including treatments for cancer, diabetes, and autoimmune diseases—are expected to lose exclusivity. Generic and biosimilar manufacturers are poised to capture massive market share—but to do so swiftly and successfully, they must get more than the molecule right.
The Unseen Race: Speed to Shelf
With a drug going off-patent, speed becomes the differentiator. The first generic to hit the shelves often captures the lion's share of the market. This "first-mover advantage" isn’t just about production capabilities—it’s also about how fast you can launch compliant, consumer-ready packaging.
This is where packaging, labeling, and artwork management (PL&A) become critical.
Where’s the Opportunity?
1. High Volume, High Complexity
- Generic drugs often operate in high-volume segments.
- Each SKU may need multiple variants depending on geography, language, dosage, regulatory format, and branding.
- Effective PL&A management helps handle this complexity at scale.
2. Regulatory Dynamics
- Labeling regulations vary widely across regions (FDA, EMA, CDSCO, etc.).
- As generics expand globally, manufacturers must ensure rapid and precise label localization and updates.
- A robust labeling system reduces regulatory risk and avoids market delays.
3. Shorter Lead Times
- Originator companies typically have long timelines to launch.
- Generics work on compressed timelines post-patent expiry.
- A fast, automated, and standardized artwork development process becomes essential to meet those tight deadlines.
The PL&A Edge: Why It Matters
Packaging
- Standardization of primary and secondary packaging for economies of scale.
- Innovation in sustainable or low-cost materials to improve margins.
- Customization for retail vs institutional sales.
Labeling
- Accurate dosage information, contraindications, and local regulatory statements.
- Real-time change management: updated leaflets, inserts, and on-pack info.
- Error-proofing with digital approval workflows.
Artwork Management
- Multi-market artwork rollouts (e.g., same drug marketed in 10+ countries).
- Version control, digital asset libraries, and multi-language management.
- Cloud-based collaboration between regulatory, marketing, and packaging teams.
Technology as an Enabler
Modern PL&A platforms offer:
- Cloud-based artwork tools with role-based approvals
- Digital label repositories to avoid duplication
- AI-driven proofreading tools to catch text, font, and layout errors
- Automated change management workflows when safety or regulatory data updates
These tools reduce human error, compress design-to-market time, and support compliance audits.
Case in Point: Biosimilars
Biosimilars, the generic equivalents of biologic drugs, come with complex storage, stability, and administration requirements. Proper packaging and labeling are crucial—not just for compliance but also for healthcare worker confidence and patient adherence.
For biosimilars entering regulated markets, packaging differentiation can even become a marketing edge—think tamper-evident designs, ergonomic prefilled syringes, or QR-code-enabled traceability.
Strategic Recommendations
For companies preparing for the patent cliff opportunity:
- Invest in PL&A platforms early – don’t treat it as post-approval activity.
- Build cross-functional teams (Regulatory + Packaging + Quality) for artwork readiness.
- Standardize packaging hierarchies where possible to reduce SKU complexity.
- Create templates for rapid artwork adaptation across geographies.
- Implement automated change management for labeling updates.
From Compliance to Competitive Advantage
In a market shaped by speed and precision, packaging, labeling, and artwork are not just regulatory checkboxes—they are strategic weapons. Companies that prepare for the patent cliff with an agile and intelligent PL&A system will not only enter the market faster but also win consumer trust, reduce compliance risk, and drive operational efficiency.
So, as we race toward a new horizon of generic and biosimilar launches, it’s time to look beyond the molecule—and see the opportunity in the label.



