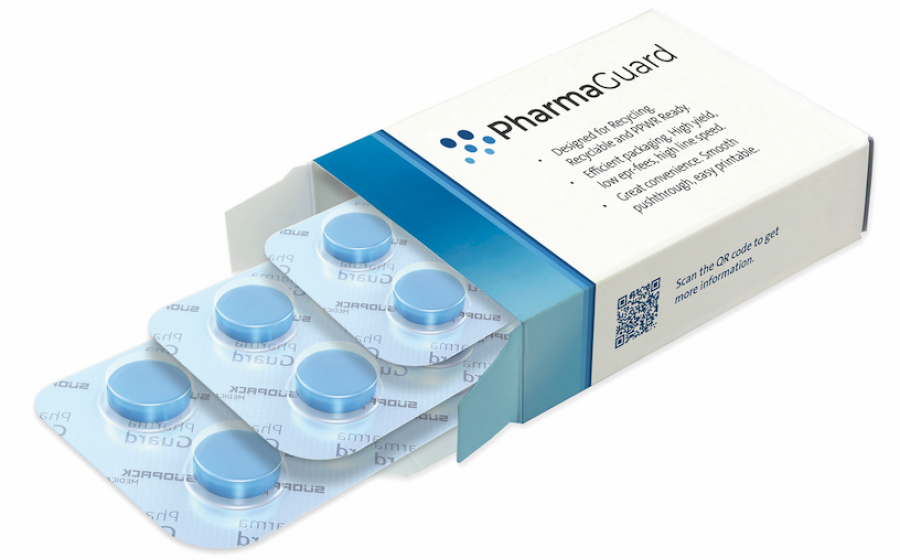
SÜDPACK MEDICA, in collaboration with IMA, is setting a new standard for sustainability in pharmaceutical packaging with its groundbreaking PharmaGuard® solution—a recyclable, polypropylene (PP)-based blister film that offers the same efficiency, protection, and reliability as conventional PVC/PVDC systems.
- Today is: