EMA has endorsed two statements about the importance, safety and effectiveness of vaccines published today by the International Coalition of Medicines Regulatory Authorities (ICMRA). International regulators from around the world have come together and jointly developed these statements for healthcare professionals and the general public to give assurance that the regulatory processes for the authorisation and safety monitoring of vaccines are robust, independent and focus firmly on public health.
‘The COVID-19 health emergency reminds us how important vaccines are to protect ourselves and our loved ones against infectious diseases,’ said Guido Rasi, Chair of ICMRA and EMA’s Executive Director. ‘In fact, vaccines are that one medical intervention that benefits not only those who receive it directly but also those who are too young, too old or too ill to be vaccinated themselves.’
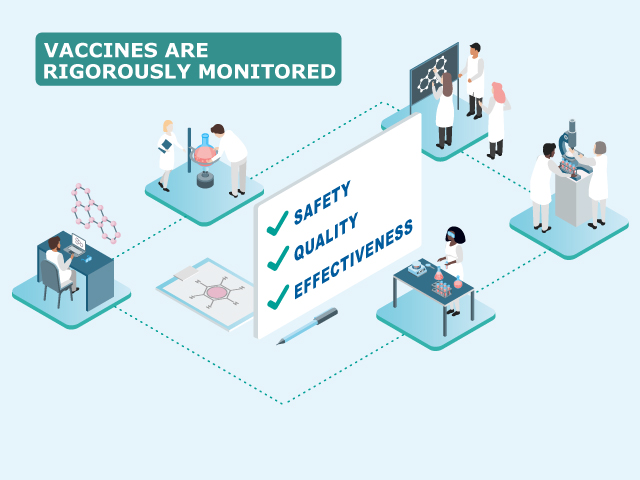
The two ICMRA statements aim to reassure healthcare professionals and the public around the globe that medicines regulators only allow vaccines onto the market that fulfil the highest standards of safety, efficacy and quality.
In recent years, vaccination coverage has dropped to sometimes dangerously low levels in some countries, which increases the risk of the disease spreading and affecting the unvaccinated. The ICMRA statements reiterate that it is everyone’s responsibility to get vaccinated in order to protect not only themselves but also their friends, communities, vulnerable populations who cannot get immunised as well as the generations to come.
ICMRA is an international coalition of 29 medicines regulatory authorities from every region in the world, with WHO as an observer, which works to address global regulatory challenges. Medicines regulators recognise their important role in facilitating access to safe and effective high-quality medicines that are essential to human health and well-being. This includes ensuring that the benefits of vaccines outweigh their risks.
The development of the joint statements followed a series of discussions among ICMRA members on new ways to collaborate and enhance confidence in vaccine safety and effectiveness. The ICMRA statements are available in multiple languages on the ICMRA website.
Contact point
EMA press office
Tel. +31 (0)88 781 8427
E-mail: press@ema.europa.eu
Follow us on Twitter @EMA_News